Battery Validation Platform for Medical Device Manufacturers
Centralize electrical, mechanical, and environmental test data to accelerate supplier qualification, support regulatory submissions, and prevent costly recalls
Comprehensive Test Type Coverage
Micantis integrates data from all battery test categories required for medical device validation
Electrical Safety & Abuse
- Short-circuit tests (room temp & high temp)
- Over-charge and forced discharge
- High-rate charging / abnormal charge
- Internal resistance and impedance spectroscopy (EIS)
- Over-discharge protection verification
Standards: IEC 62133, UL 1642, UL 2054
Mechanical Tests
- Vibration and mechanical shock
- Crush and impact tests
- Drop tests (pack and enclosure)
- Mold stress-relief / enclosure integrity
- Acceleration tests
Standards: IEC 62133, UL 1642, UL 2054, UN 38.3
Environmental Tests
- Thermal abuse / high-temperature exposure
- Temperature cycling
- Altitude simulation / low pressure
- Humidity exposure
- Fire exposure tests
Standards: UL 1642, UL 2054, IEC 62133, UN 38.3
How Micantis Solves Medical Device Battery Testing Challenges
Unified Multi-Domain Test Platform
Create a "Digital Battery Passport" linking all test data to each cell and lot
Integrate electrical, mechanical, environmental, and supplier data into one unified system. Every test result linked to individual cells and lots with complete traceability.
- Electrical tests: OCV, impedance, cycle life, safety abuse tests
- Mechanical tests: Drop, crush, vibration, shock, impact
- Environmental tests: Thermal, altitude, humidity, temperature cycling
- Supplier documentation: Certificates of compliance, analysis reports

Statistical Process Control for Sample-Based Testing
Make confident lot acceptance decisions from small sample sizes
Destructive tests require sampling. Micantis ensures your sample data is statistically valid with automated analysis and confidence scoring.
- Automated statistical analysis: Confidence intervals, control charts, Z-scores
- Anomaly detection: Flag statistically unusual lots even if they pass specs
- Trend analysis: Track supplier consistency over time
- Risk scoring: Compare each lot against historical baseline
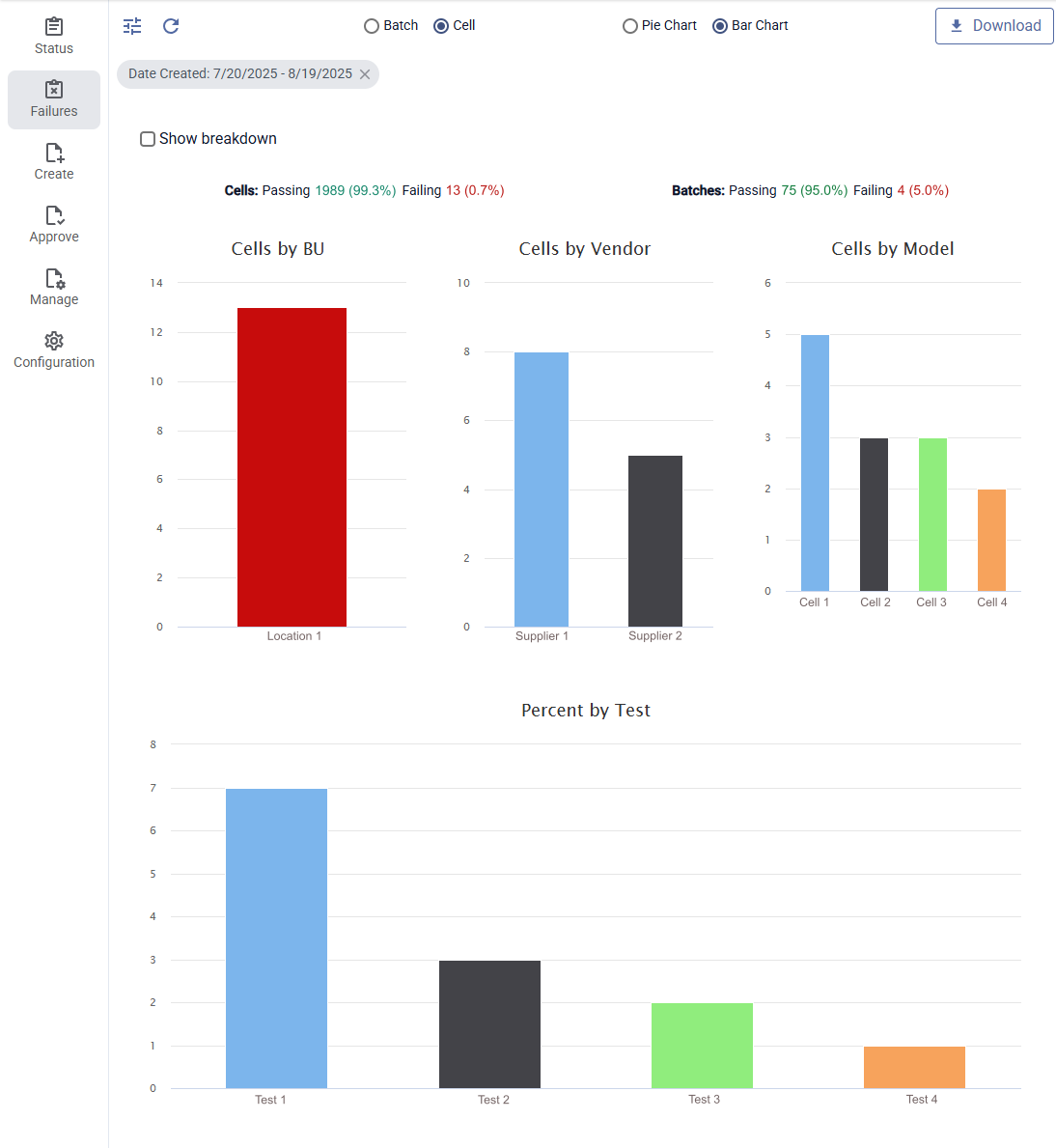
Customizable Reporting for Regulatory Submissions
Compile comprehensive battery validation data for Design History Files
Micantis centralizes your data and generates professional reports ready for regulatory review.
- Customizable templates: Match your documentation requirements
- Automated compilation: Pull data from all test types into unified reports
- Multiple formats: PDF, Excel, PowerPoint exports
- Statistical summaries: Charts and analysis included automatically
- Version tracking: Complete audit trail
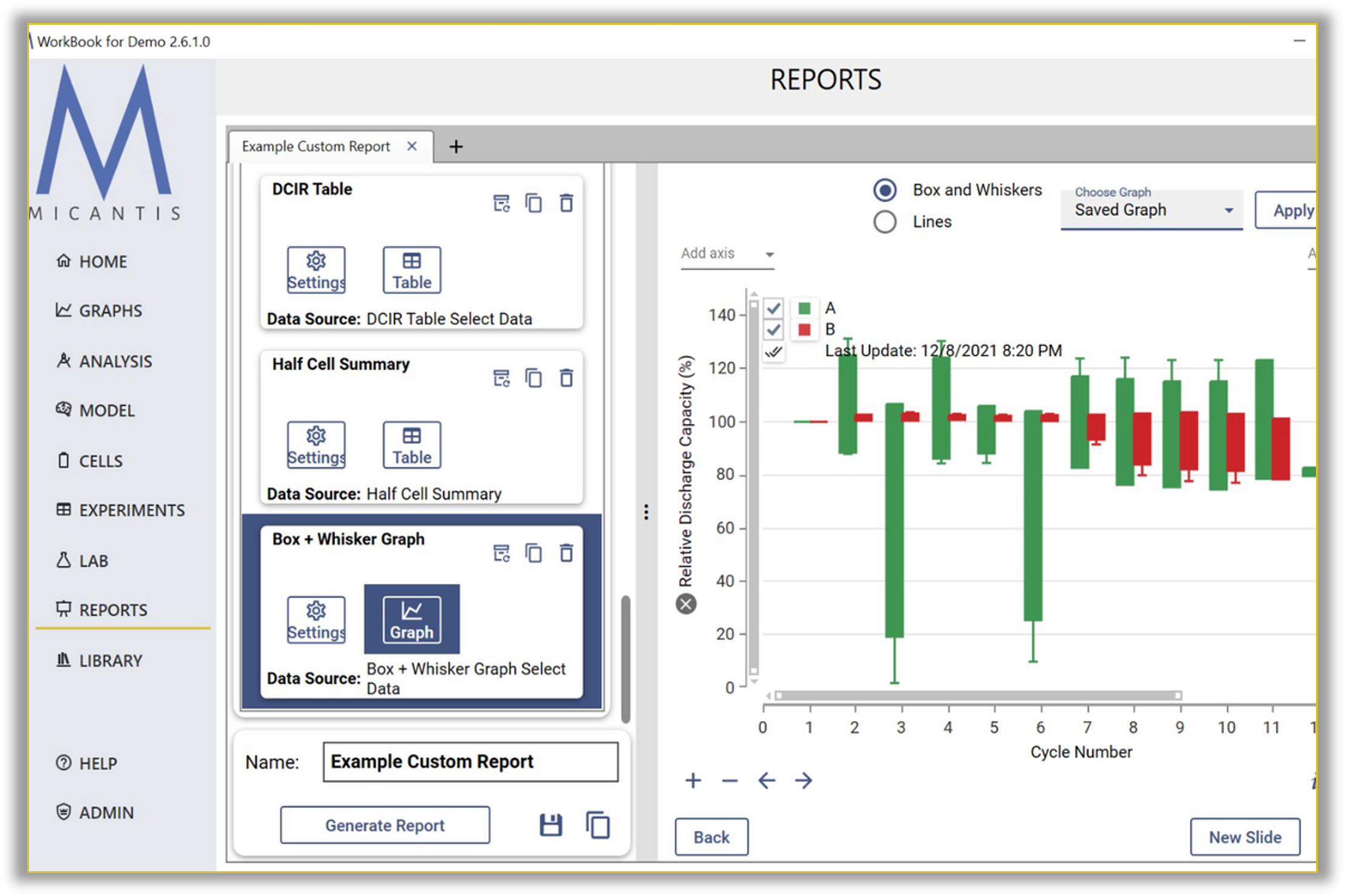
Complete Traceability for Regulatory Requirements
Chain of custody from supplier lot to device serial number
Micantis automatically links all test data throughout the product lifecycle for instant recall impact analysis.
- Automated traceability: Link all test data to device serial numbers
- Instant recall analysis: Identify all devices with specific battery lots
- Bidirectional drill-down: Device → lot → test history → supplier cert
- Time-stamped records: Full audit trail for compliance
Traceability Chain
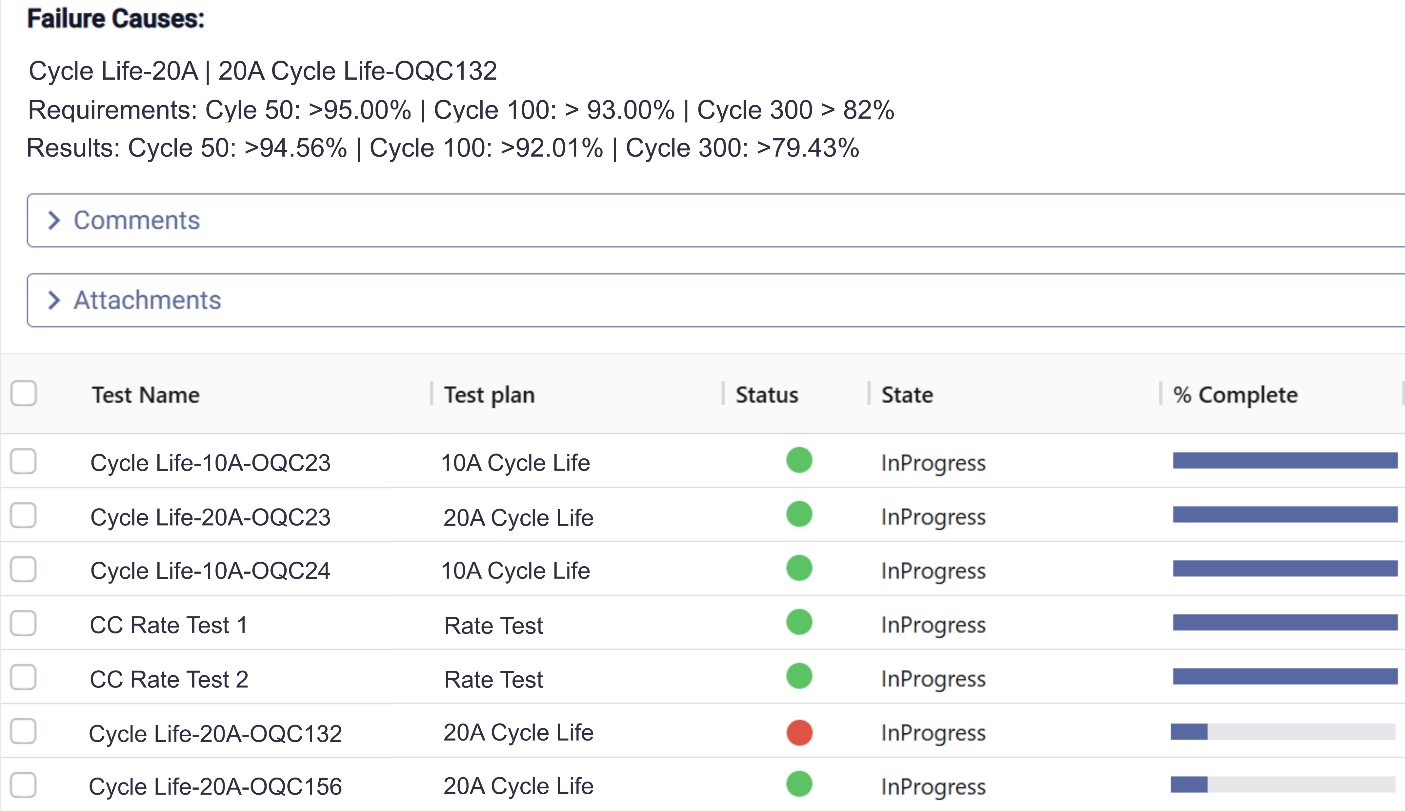
Intelligent Lot Acceptance Decision Support
Consistent, data-driven accept/reject decisions in minutes
Micantis automates acceptance decisions with intelligent scoring across all test types and clear recommendations.
- Automated pass/fail: Check compliance across all test domains
- Intelligent risk scoring: Statistical analysis and historical baseline comparison
- Multi-domain analysis: Consider electrical, mechanical, and environmental together
- Clear recommendations: Pass, fail, or flagged for review
- Complete audit trail: Document rationale for every decision
Trusted Across Medical Device Categories
Implantable Devices
Life-sustaining devices where battery failure is catastrophic
- Pacemakers and ICDs (implantable cardioverter-defibrillators)
- Neurostimulators (spinal cord, deep brain stimulation)
- Drug delivery pumps (implantable infusion systems)
- Cochlear implants
Key Challenge: Zero tolerance for failures. Hermetic seals, biocompatibility, and 5-10 year lifespan validation required.
Wearable Monitors & Sensors
Continuous monitoring devices worn by patients
- Continuous glucose monitors (CGM)
- Wearable ECG and heart monitors (Holter monitors, event recorders)
- Pulse oximeters and vital sign monitors
- Wearable insulin pumps
Key Challenge: Shelf life validation (2-5 years), self-discharge control, consistent lot quality at scale.
Portable Medical Equipment
Battery-powered equipment used in hospitals and emergency care
- Portable ultrasound machines
- Infusion pumps and patient-controlled analgesia (PCA) pumps
- Automated external defibrillators (AEDs)
- Portable ventilators and oxygen concentrators
- Patient monitors (bedside and transport)
Key Challenge: High reliability for emergency use, wide temperature range, ruggedization.
Surgical Power Tools
Battery-powered instruments for surgical procedures
- Powered surgical staplers and cutters
- Bone drills and saws (orthopedic surgery)
- Electrosurgical generators (portable units)
- Powered retractors and endoscopic tools
Key Challenge: High power discharge, sterilization compatibility, consistent performance across battery life.
How Micantis Supports Testing Requirements
Micantis helps you manage comprehensive battery testing across all required standards
Test Data Organization:
Centralize all IEC 62133, UL 1642, and UL 2054 test results in one platform with complete traceability.
Incoming Inspection:
Document IQC procedures and results for every lot. Statistical acceptance criteria with automated pass/fail decisions.
Complete Traceability:
Chain of custody from supplier lot → IQC → verification testing → device serial number. Instant recall impact analysis.
Multi-Domain Testing:
Link electrical tests (cycle life, OCV, impedance) with mechanical tests (drop, crush, vibration) and environmental tests (thermal, altitude) for comprehensive validation.
Risk Analysis Support:
Data-driven hazard analysis. Track battery failure modes and mitigation controls with statistical evidence.
Supplier Management:
Document supplier qualification, ongoing monitoring, and change control. Performance trend reports.
Measurable Impact on Medical Device Programs
75%
Faster Supplier Qualification
Reduce from 12 months to 2-3 months using predictive cycle life models and automated comparison reports.
80%
Less Documentation Time
Compile comprehensive test reports in days instead of weeks. All data accessible in one system.
90%
Faster Root Cause Analysis
Instantly correlate field failures back to specific test anomalies. Complete traceability from device SN to all test data.
$5M-$50M
Recall Prevention
Catch defective lots before device assembly through ML-powered anomaly detection and cross-domain correlation.
Additional Business Benefits
- Reduce testing costs: Optimize which cells get expensive destructive tests using predictive risk scoring
- Faster time-to-market: Accelerated battery validation enables faster product launches
- Supplier leverage: Data-driven negotiations based on objective performance comparisons
- Warranty cost reduction: Early detection of capacity fade and premature failures
- Regulatory confidence: Comprehensive documentation simplifies submissions
Engineering Productivity Gains
- Automated data compilation: No more hunting through folders for test results
- Consistent decision-making: Standardized acceptance criteria across teams and sites
- Instant historical comparisons: "Has this happened before?" answered in seconds
- Focus on high-value work: Engineers analyze trends, not spreadsheets
- Institutional knowledge capture: Correlation models preserve tribal knowledge
Frequently Asked Questions
Q: Does Micantis replace our battery cyclers or test equipment?
A: No. Micantis integrates with your existing equipment (battery cyclers, impedance testers, thermal chambers, drop test rigs, etc.). We automate the data analysis, correlation, and reporting—you keep using the equipment you already have.
Q: How does cross-domain correlation work?
A: Micantis links all test data (electrical, mechanical, environmental) to the same cell/lot ID. Our ML algorithms analyze historical data to discover patterns like "cells with >15mΩ impedance fail drop tests 40% more often." You can then use these insights to optimize IQC screening and predict which lots need extra scrutiny.
Q: Can we import historical test data?
A: Yes. We support bulk import of historical data from Excel, CSV, and cycler file formats. This allows immediate correlation analysis using your existing dataset.
Q: What test equipment brands do you integrate with?
A: We support automated import from:
- Battery Cyclers: Arbin, Maccor, Bitrode, PEC, Digatron, Neware, BaSyTec
- Impedance Testers: Hioki, Arbin BT-2000, custom EIS equipment
- Thermal Chambers: Espec, Thermotron, Cincinnati Sub-Zero
- Generic: CSV, Excel, text files from mechanical test equipment
Q: How accurate is the predictive cycle life model?
A: Our AI models achieve 99.7% accuracy predicting end-of-life cycle count from 50-100 cycles. Models are trained on millions of battery test results across NMC, NCA, LFP, and other chemistries. Predictions are validated against actual cycle life data.
Q: Can we customize reports for our specific needs?
A: Yes. Micantis provides flexible report templates that you can customize to match your documentation requirements. Export to PDF, Excel, or PowerPoint in formats that work for your regulatory submissions.
Q: Can we track batteries from multiple suppliers?
A: Absolutely. Micantis manages multi-supplier scenarios with side-by-side performance comparisons, supplier-specific baselines, and automated alerting when supplier performance drifts.
Q: How long does implementation take?
A: Most customers are operational within 2-4 weeks:
- Week 1: Data integration setup with your test equipment
- Week 2: User training and specification configuration
- Week 3-4: Pilot testing and report template customization
- Ongoing: Dedicated support and continuous improvement
Q: Can we customize acceptance criteria and risk scoring?
A: Yes. You define specifications, risk weights, and acceptance rules for each test type. Micantis applies these consistently across all lots and provides recommendations based on your criteria.
Q: What if we have both primary and rechargeable batteries?
A: Micantis supports both primary (non-rechargeable) and rechargeable battery testing. We handle different standards (IEC 60086 vs. IEC 62133) and test protocols for each battery type.
Ready to Accelerate Your Medical Device Battery Program?
Join medical device manufacturers who are reducing supplier qualification time, streamlining regulatory submissions, and preventing costly recalls with Micantis.
Schedule a Demo
See how medical device companies use Micantis to:
- Integrate electrical, mechanical, and environmental test data
- Discover cross-domain correlations that catch problems earlier
- Accelerate supplier qualification from 12 months to 2-3 months
- Compile comprehensive reports for regulatory submissions
- Maintain complete traceability from lot to device serial number
Talk to a Battery Testing Expert
Have specific questions about your battery testing workflow? Our team includes battery engineers with medical device experience who can discuss:
- IEC 62133, UL 1642, and UL 2054 testing strategies
- Regulatory submission data requirements
- Statistical approaches for sample-based acceptance testing
- Predictive models for shelf life and cycle life validation
From supplier qualification to regulatory submissions to production quality control—Micantis supports your entire medical device battery journey.