From Theory to Practice: Exploring How a Potentiostat Operates
Quick Answer: A potentiostat is an instrument used in electrochemistry to control and measure the electrical potential between a working electrode and a reference electrode in a solution. It ensures the potential remains stable, allowing scientists and engineers to monitor and analyze electrochemical reactions precisely.
Electrochemistry sits at the heart of countless innovations, especially in battery design and analysis. Understanding the behavior of batteries under different conditions isn't just beneficial; it's essential. This is where a potentiostat comes into play—an unsung hero in the electrochemical research and development arena.
At its core, a potentiostat is a device that controls the voltage difference between a working electrode and a reference electrode. What it essentially does is maintain this voltage at a constant level, regardless of what's happening in the rest of the system. This control allows for precise experiments, leading to useful data about how different materials react under various electrical conditions.
Moreover, for battery designers and engineers, grappling with slow R&D cycles and high development costs, mastering the potentiostat's capabilities can be a game changer. It enables the fast optimization of battery designs, helps to weed out ineffective prototypes early on, and streamlines testing and analysis—accelerating time to market while cutting overhead.
In the subsequent sections, we will dive deeper into the workings of potentiostats, their core components, and how they drive essential electrochemical experiments—illuminating paths for battery innovation.
Understanding the Potentiostat
Imagine having a remote control that can precisely adjust how much energy flows in a science experiment. That's essentially what a potentiostat does in electrochemistry. Let's break this down into simpler terms to understand how a potentiostat works, focusing on its electronic hardware, the setup of a three-electrode cell, and how it manages voltage control.
Electronic Hardware
A potentiostat is like the brain of electrochemical experiments. It's a piece of electronic hardware that makes sure everything runs smoothly. Inside this "brain," there are circuits, much like the ones you find in your computer or phone, but these are specially designed to control electrochemical reactions. The heart of these circuits is something called an operational amplifier (op-amp for short). This op-amp is crucial because it helps adjust the flow of electricity to keep the experiment running just right.
Three-Electrode Cell
Now, let's introduce the players in our experiment. In a typical setup, we have three main electrodes:
The Working Electrode (WE): This is where the action happens. It's like the stage for our chemical reactions.
The Reference Electrode (RE): This electrode is the ruler of the setup. It sets a steady point that the potentiostat can use to measure everything else against.
The Counter (Auxiliary) Electrode (CE): Think of this as the support character. It helps complete the circuit and allows electricity to flow properly.
This trio works together under the watchful eye of the potentiostat to explore how materials react under different electrical conditions.
Voltage Control
The magic of a potentiostat lies in its ability to control voltage with precision. Here's a simple way to picture it:
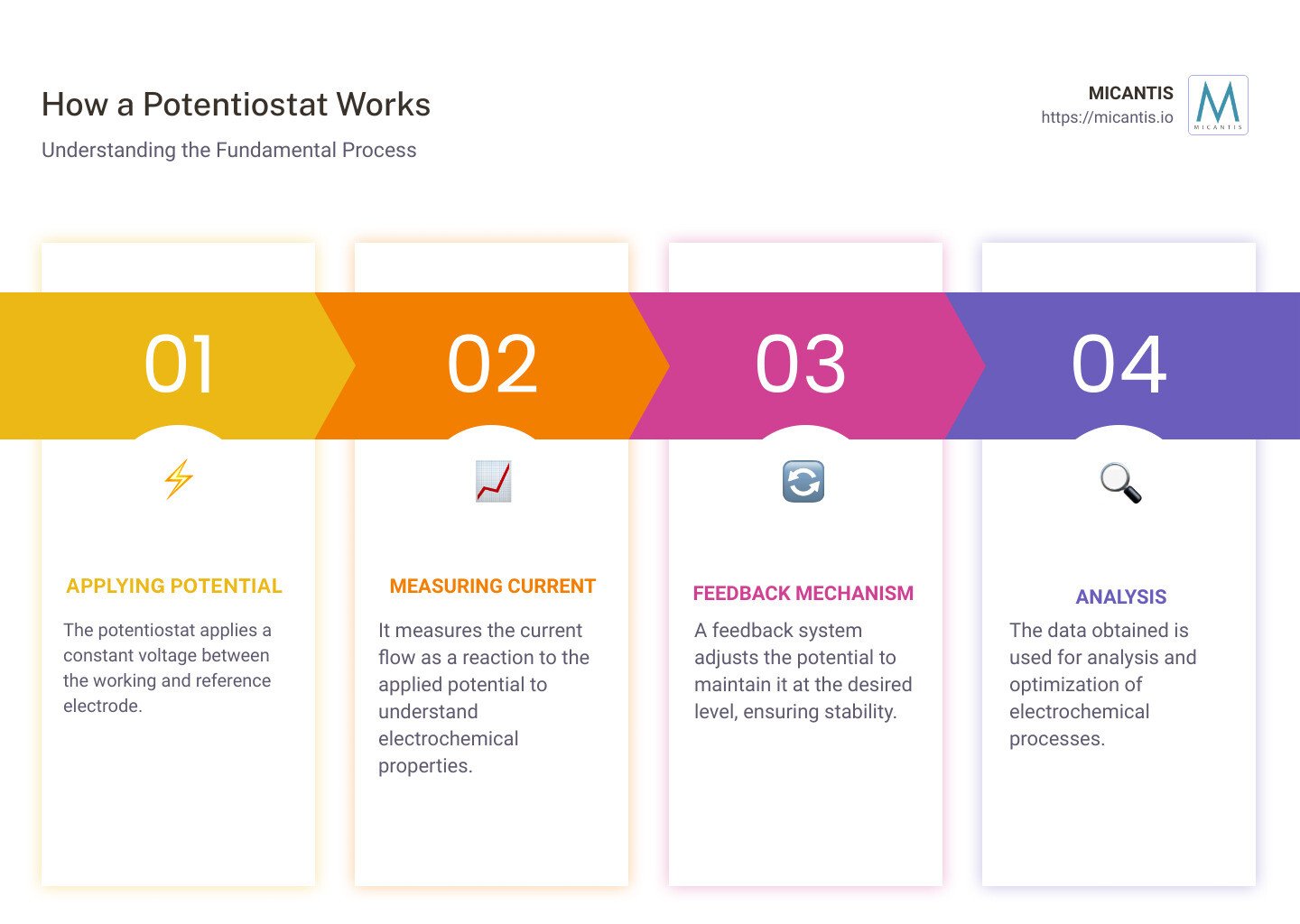
Applying Potential: The potentiostat decides how much voltage to apply. It's like setting the volume on your music player — not too loud, not too quiet, just right for your experiment.
Measuring Current: As the experiment runs, our potentiostat keeps an eye on how much current is flowing. It's continuously checking to make sure the "music" plays smoothly.
Feedback Mechanism: This is where the potentiostat shines. If anything starts to drift away from the plan (like the music getting too loud), the potentiostat adjusts the voltage to bring everything back in line. This feedback loop is crucial for keeping the experiment on track.
In conclusion, understanding a potentiostat boils down to seeing it as a masterful conductor, orchestrating the flow of electricity in a way that lets scientists explore the mysteries of materials at a molecular level. With its sophisticated electronic hardware, the strategic setup of a three-electrode cell, and precise voltage control, the potentiostat paves the way for groundbreaking discoveries in electrochemistry.
We'll delve into the core components of a potentiostat and how it becomes an indispensable tool in electrochemical experiments, especially in the realm of battery innovation, where companies like Micantis are leading the charge.
Core Components of a Potentiostat
When we talk about how a potentiostat works, it's like telling the story of a concert where each instrument plays a crucial role. In electrochemistry, the potentiostat is the conductor, and its components are the musicians. Let's meet them!
Working Electrode
Imagine you're painting on a canvas. The Working Electrode is like your brush. It's where the action happens. This electrode can be made of "inert" materials like gold or platinum, which don't react easily. This is great for experiments because it means they won't mess with the results.
Inert materials: These are the unsung heroes, providing a stable surface for reactions without participating in them.
Corrosion testing: When it comes to testing how materials rust or corrode, the working electrode steps in as a mini-version of the metal in question.
Battery connection: In battery studies, this electrode connects directly to either the battery's anode or cathode, playing a key role in understanding how batteries charge and discharge.
Reference Electrode
Think of the Reference Electrode as a reliable friend who always tells you the truth. It keeps the working electrode in check, making sure the voltage stays constant. It's the benchmark.
SCE and Ag/AgCl: These are types of reference electrodes. They're like the gold standard in the lab, providing a steady point of comparison.
Constant potential: This is their superpower. Even when things get electrically noisy, they keep their cool, ensuring accurate measurements.
Counter (Auxiliary) Electrode
The Counter Electrode is the wingman. It completes the circuit, allowing current to flow smoothly through the system. Without it, you'd have an open circuit, and no reactions could happen.
Circuit completion: It's all about teamwork. This electrode makes sure the electrical circuit is closed so everything can work as it should.
Inert conductor: Often made from materials like platinum, it stands by, ensuring the current has a path to flow without getting involved in the reactions.
Electrochemical Cell
The Electrochemical Cell is the stage where all these components come together. It includes:
Electrolyte: This is the medium that allows ions to move freely, carrying charges between the electrodes. Think of it as the crowd that transfers the energy from the stage to the rest of the concert hall.
Electrochemical reactions: These are the performances, the main events that we're all here to see. They happen at the surface of the working electrode.
Cell circuit: This is the layout of the concert. It connects all the components, making sure the show can go on.
In this setup, each part plays a vital role. The working electrode is where the reactions get their spotlight, the reference electrode keeps the performance honest, and the counter electrode supports the whole show. The electrolyte in the cell ensures that the current can flow, allowing the electrochemical reactions to unfold. It's a finely tuned orchestra that, when conducted by a skilled researcher, can lead to groundbreaking discoveries in fields like battery technology and corrosion prevention.
As we transition from understanding the components to seeing them in action, it's clear that the potentiostat is more than just a piece of equipment. It's a gateway to innovation, enabling scientists and companies like Micantis to push the boundaries of what's possible in electrochemistry.
How a Potentiostat Works
Diving into the workings of a potentiostat, we uncover the magic that makes it essential in electrochemistry. Let's break it down into three main actions: applying potential, measuring current, and the feedback mechanism. This trio works in concert to ensure the potentiostat does its job effectively.
Applying Potential
At its core, a potentiostat acts as a sophisticated voltage source. It's designed to vary its output potential to keep the experiment on track. Think of it like a smart thermostat for your home, but instead of controlling temperature, it's all about managing voltage across an electrochemical cell.
The principle here is pretty straightforward and follows Ohm’s Law. If you've ever tinkered with electronics, you know Ohm's Law is about the relationship between voltage, current, and resistance. In the context of a potentiostat, it adjusts the voltage to maintain a stable environment for the electrochemical reaction, despite any changes in resistance.
Measuring Current
Now, let's talk about current flow. The potentiostat keeps a keen eye on the current that dances between the working and counter electrodes. This is where the action happens, revealing the electrochemical properties of the material being tested. It's like measuring the heartbeat of the electrochemical reaction, ensuring everything is proceeding as expected.
Feedback Mechanism
The real hero in this story is the negative feedback mechanism. This is what allows a potentiostat to adjust and maintain the desired potential with precision. It constantly compares the actual potential to the desired one and makes corrections to keep things stable. This ensures voltage stability, which is crucial for the accuracy of the experiment.
Imagine driving a car with cruise control. You set your desired speed, and the car adjusts the throttle to maintain that speed, slowing down or speeding up as needed. The potentiostat does something similar but with voltage in an electrochemical cell.
This feedback loop is vital. Without it, the potential could drift due to changes in the cell (like resistance changes as the reaction progresses), potentially skewing the results. The feedback mechanism ensures that the potential remains constant, providing reliable and accurate data.
In summary, a potentiostat works by applying a specific potential to an electrochemical cell, measuring the resulting current flow, and using a feedback mechanism to adjust and maintain the voltage stability. This trio of actions allows scientists and researchers to explore the electrochemical properties of materials with precision, leading to breakthroughs in fields like battery technology and corrosion prevention.
As we've seen, the potentiostat is not just a piece of equipment. It's a precision tool that plays a crucial role in advancing our understanding of electrochemical processes. Whether it's in academic research or industrial applications, the potentiostat is key to unlocking new possibilities in electrochemistry, making it an indispensable tool for innovators and companies like Micantis.
Potentiostat in Electrochemical Experiments
Electrochemical experiments are where the rubber meets the road, transforming theory into tangible insights. Two techniques stand out for their ability to unravel the mysteries of electrochemical reactions: Cyclic Voltammetry and Linear Sweep Voltammetry. Let's dive into how these methods work and why they're so crucial.
Cyclic Voltammetry
Imagine you're on a rollercoaster that loops back on itself, offering views of the landscape at different speeds and angles. This is akin to Cyclic Voltammetry (CV), a technique that takes us on a thrilling ride through the ups and downs of electrochemical reactions.
Redox Events: CV is all about observing how substances gain or lose electrons (redox events). It's like watching a dance of molecules, where each step and turn reveals more about their behavior and interactions.
Electrochemical Reversibility: Through CV, we can tell if a reaction can easily reverse itself or if it's a one-way street. It's akin to checking if our rollercoaster can go backward as smoothly as it goes forward, offering insights into the reaction's flexibility.
Energy Levels: CV helps us peek into the energy levels of materials, especially semiconducting polymers. It's like understanding the potential energy stored in the rollercoaster at various points, predicting how it will perform under different conditions.
Linear Sweep Voltammetry
Now, envision a scenic tram ride up a hill, steadily increasing in elevation. This is the essence of Linear Sweep Voltammetry (LSV), where we gradually change the voltage and observe the resulting current flow.
Potential Delivery: In LSV, we control the voltage applied to the working electrode, slowly ramping it up. It's a methodical approach, allowing us to see how the electrochemical system responds to a steady increase in potential.
Working Electrode Focus: The spotlight is on the working electrode, where the oxidation or reduction happens. It's like focusing our binoculars on specific landmarks during our tram ride, giving us detailed views of the reactions occurring there.
Electrochemical Analysis: LSV is a powerful tool for analyzing unknown substances and determining solution concentrations. It's like using a detailed map to navigate our tram ride, understanding the terrain and its features better.
Both Cyclic Voltammetry and Linear Sweep Voltammetry are essential in exploring the electrochemical landscape. They allow us to understand redox events, assess electrochemical reversibility, and determine energy levels with precision. Through potential delivery and a focus on the working electrode, these techniques offer deep insights into electrochemical analysis.
Just as a piece of equipment is more than its physical form, these experiments are more than their procedures. They are gateways to understanding the fundamental processes that drive electrochemistry forward. For companies like Micantis, mastering these techniques is not just about conducting experiments; it's about paving the way for innovation and discovery in the realm of electrochemistry.
Frequently Asked Questions about Potentiostats
As we dive into electrochemistry, we encounter tools that are pivotal in pushing the boundaries of research and development. Among these tools, the potentiostat stands out as a cornerstone for a wide array of experiments. Let's unravel some of the most common queries surrounding this instrument, simplifying the complex in the spirit of discovery.
How does a potentiostat apply potential?
Imagine you're holding a remote control, and with a press of a button, you can adjust the brightness of your TV screen. A potentiostat works in a somewhat similar fashion but in the realm of electrochemistry. It's like a precise remote control for the potential (voltage) applied across an electrochemical cell.
When an experiment begins, the potentiostat sends out a specific voltage to the working electrode. This is not just any random voltage; it's carefully chosen based on what the scientist wants to study. The potentiostat keeps this voltage steady, allowing the researcher to observe how the electrochemical reactions change under these conditions. It's a bit like adjusting the brightness to see better details on your TV screen.
What is the basic principle of a potentiostat?
At its heart, a potentiostat is all about balance and control. Think of it as a tightrope walker, where the rope is the potential difference between two electrodes, and the walker is the current flowing through the cell. The potentiostat's job is to keep the walker balanced by adjusting the rope's tension (the potential) so that the walker (current) can move smoothly across without falling.
This balance is achieved through a feedback loop involving an operational amplifier. The potentiostat continuously compares the actual potential to the desired potential. If there's a discrepancy, it adjusts the current to bring things back in line. This ensures that the experiment proceeds under the exact conditions set by the researcher.
What is the connection of a potentiostat?
Connecting a potentiostat to an electrochemical cell is akin to setting up your gaming console to your TV. There are specific cables and ports where everything needs to be plugged in for the system to work correctly.
A typical setup involves three electrodes: the working electrode (where the reaction of interest happens), the reference electrode (which acts like a stable voltage source for comparison), and the counter electrode (which completes the circuit, allowing current to flow). The potentiostat connects to all three through a cell cable. This setup allows the potentiostat to control the potential across the cell while monitoring the current that flows as a result of electrochemical reactions.
This trio of connections is critical. Without the reference electrode's stable potential for comparison, controlling the working electrode's potential would be like trying to hit a moving target. And without the counter electrode, there would be no path for the current to flow, making the experiment impossible.
As we segue into the conclusion, it's clear that understanding how a potentiostat operates is fundamental in leveraging its capabilities for electrochemical research. Whether it's for studying battery efficiency, corrosion, or the principles of redox chemistry, the potentiostat is a gateway to deeper insights and innovations in the field. With companies like Micantis at the forefront, the potential for breakthroughs in electrochemistry is boundless, promising exciting advancements for our future.
Conclusion
Electrochemistry sits at the crossroads of innovation, powering the batteries that energize our world and safeguarding the materials that our infrastructure depends on. At the heart of this scientific frontier is the potentiostat, a tool that might seem complex at first glance but is essential for peeling back the layers of electrochemical mysteries. Understanding how does a potentiostat work is not just about grasping the mechanics of the device itself but appreciating its role in the broader context of scientific discovery and application.
In the realm of battery testing, the potentiostat is indispensable. It allows researchers to simulate and analyze the charge and discharge cycles of batteries, providing invaluable data on efficiency, longevity, and potential for improvement. This is where the synergy between practical application and theoretical research becomes most evident. By applying the principles of electrochemistry through the use of potentiostats, we can push the boundaries of what's possible in battery technology, making strides toward more sustainable and efficient energy storage solutions.
At Micantis, we're proud to stand at the intersection of theory and practice. Our commitment to advancing electrochemical research goes beyond providing cutting-edge tools and extends into a partnership with the scientific community. We understand that the future of energy, from how we power our vehicles to how we store renewable energy, hinges on the breakthroughs made in laboratories around the world today.
Our role is to equip those at the forefront of electrochemical research with the technology and support they need to turn theoretical possibilities into practical realities. Whether it's through facilitating more accurate battery testing, offering predictive modeling to streamline the research process, or simply demystifying the intricacies of how a potentiostat operates, our goal is clear. We aim to empower researchers and innovators to unlock the full potential of electrochemistry, paving the way for a future powered by cleaner, more efficient, and more reliable energy sources.
In conclusion, the journey from electrochemical theory to practical battery solutions is complex but deeply rewarding. With tools like the potentiostat and the support of companies like Micantis, researchers have what they need to explore uncharted territories in electrochemistry. The possibilities are as vast as our collective imagination and dedication to progress. Let's continue to push the boundaries together, exploring how the fundamental principles of electrochemistry can lead to innovations that power our world more sustainably and efficiently.