Understanding Potentiostats: The Ultimate Guide
Understanding Potentiostats: The Ultimate Guide
If you're diving into the exciting world of electrochemistry, biochemistry, sensor development, or particularly, battery research, knowing the ins and outs of a potentiostat is indispensable. At its core, a potentiostat is an electrochemical workhorse that lets researchers and engineers control and measure the electrical characteristics of materials in a precise and sophisticated manner.
In short, a potentiostat:
Controls the voltage across an electrochemical cell.
Measures the current response due to this controlled voltage.
Supports a wide range of electrochemical techniques, critical in fields from biochemistry to battery optimization.
Whether you're a battery designer grappling with the challenges of slow R&D cycles and high development costs, or an engineer at an EV manufacturer focused on quality testing, a deeper understanding of potentiostats could be the key to streamlining your processes.
By harnessing the capabilities of a potentiostat, you have the potential to accelerate time to market, optimize battery designs more effectively, and ultimately, pave the way for more sustainable and efficient power solutions. Let's dive into how this powerful tool plays a pivotal role in various scientific adventures, from uncovering the mysteries of electrochemical reactions to fine-tuning the batteries that power our future.
This introduction sets the stage for a fascinating journey through the fundamentals of potentiostats, how they work, and their wide array of applications in advancing technology and sustainability.
What is a Potentiostat?
Imagine you're a scientist in a lab, looking to unlock the secrets of chemical reactions, or maybe you're an engineer trying to create the next generation of batteries. There's one tool that's going to be your best friend on this journey: the potentiostat.
What's a Potentiostat? In simple terms, a potentiostat is like a very sophisticated remote control for electrochemical experiments. It's a piece of electronic hardware that allows scientists to precisely control and measure the voltage and current in an electrochemical cell—a tiny environment where chemical reactions can be sparked by electricity.
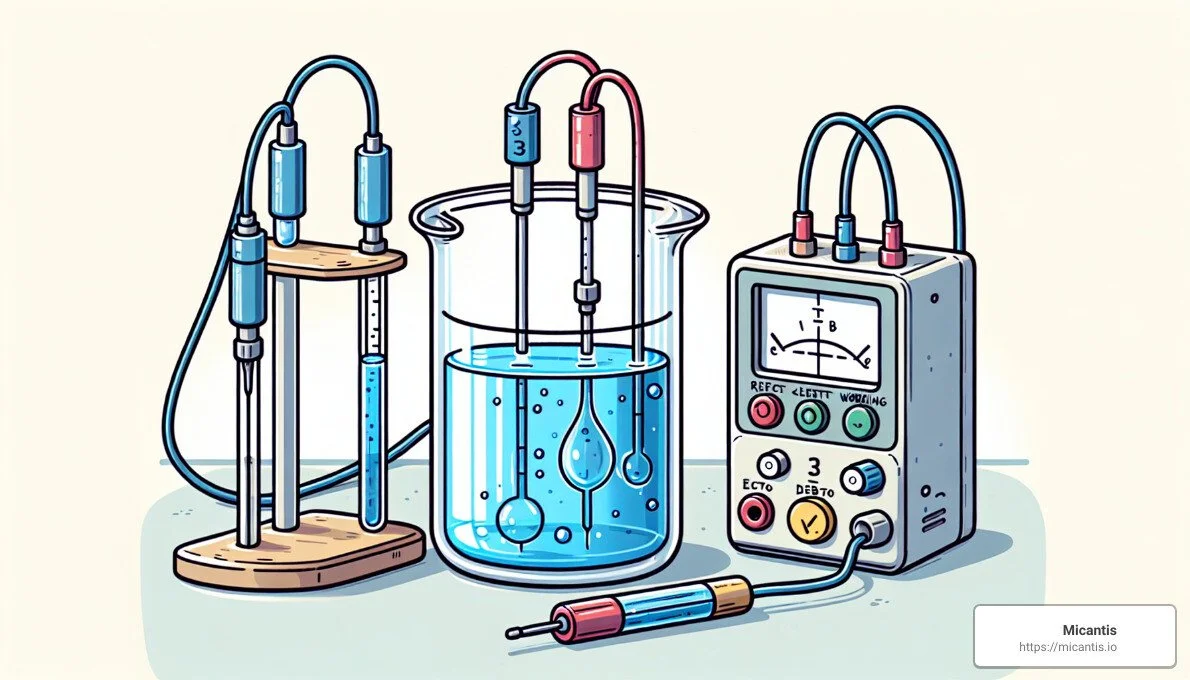
The Three-Electrode Cell At the heart of these experiments is something called a three-electrode cell. This setup includes:
The Working Electrode (WE): This is where the magic happens—chemical reactions are either started or stopped here.
The Reference Electrode (RE): This is the "gold standard" electrode that helps keep the working electrode's voltage steady.
The Counter (Auxiliary) Electrode (CE): This electrode completes the circuit, allowing the electricity to flow.
Why Use a Potentiostat? Using a potentiostat, scientists can dive into electroanalytical experiments. These experiments help us understand how chemicals react under certain electrical conditions. For instance, by tweaking the voltage at the working electrode and measuring how the current changes, researchers can figure out how quickly a chemical reaction happens or how much of a certain chemical is present in a solution.
Real-World Impact Think about a world where batteries charge in minutes and last for weeks, or where we can quickly detect harmful chemicals in drinking water. That world isn't as far off as you might think, thanks to the experiments potentiostats make possible.
In Summary: A potentiostat is more than just a piece of lab equipment. It's a gateway to understanding the electrochemical processes that drive innovation in energy storage, environmental protection, and much more. Through the precise control and measurement capabilities of the potentiostat, scientists and engineers can explore, experiment, and eventually engineer solutions that make our world a better place.
As we continue to delve into the applications and nuances of potentiostats, these devices are at the forefront of bridging the gap between theoretical chemistry and practical, life-changing technologies.
How Does a Potentiostat Work?
When we talk about a potentiostat, we're diving into the heart of electrochemistry. But how does this crucial piece of equipment operate? Let's break it down into simple terms.
Negative Feedback System
Imagine you're riding a bike up a hill, and you want to maintain a steady speed. If you start to slow down, you pedal harder. If you're going too fast, you ease off. This is similar to how the negative feedback system in a potentiostat works.
In more technical terms, the potentiostat constantly compares the electric potential (voltage) it's supposed to maintain with the actual potential at the working electrode. If there's a difference, it adjusts the current to bring the potential back to the desired level. This ensures stable conditions for the electrochemical reaction being studied.
Electrode Potential Control
Controlling the electrode potential is like setting the exact temperature you want on your air conditioner. You don't just want it to be cooler; you want it at a specific temperature. Similarly, in an electrochemical experiment, you don't just want a reaction to happen. You want it to happen under controlled conditions.
The potentiostat does this by applying a specific voltage between the working electrode (where the reaction occurs) and the reference electrode (which acts like our thermometer, telling us the current "temperature" or, in this case, potential). This precise control allows scientists to observe how reactions occur at different potentials, leading to deeper insights into the materials or processes being studied.
Current Measurement
Now, imagine you're filling a pool with water and you want to know how much water it takes to fill it up. You could measure the flow of water into the pool over time. In a similar way, the potentiostat measures the flow of electric current between the working and counter electrodes as the reaction proceeds. This "flow" of electrons gives us valuable information about the electrochemical reaction, like how fast it's happening and how much charge is being transferred.
By keeping a close eye on this current, the potentiostat helps scientists understand the kinetics of the reaction — essentially, it tells us how the reaction moves and changes over time.
In Summary:
The potentiostat is like a sophisticated control system for electrochemical experiments. It uses a negative feedback system to maintain a desired potential, accurately controlling the electrode potential to ensure precise conditions for the reaction. At the same time, it measures the current to provide insights into the reaction's behavior. This trio of actions allows for a deep exploration of electrochemical processes, laying the groundwork for advancements in fields like battery development, corrosion prevention, and much more.
Keep in mind that the potentiostat isn't just a tool; it's a window into the molecular dance of electrons that power our world. Next, we'll explore the various applications of potentiostats, from the lab bench to industrial settings, and see how they're shaping the future of technology.
Applications of Potentiostats
When we talk about potentiostats, we're diving into a world where the tiny movements of electrons can tell us huge stories about the materials and reactions they're part of. Let's break down how potentiostats play a crucial role in fields like corrosion research, chemical synthesis, battery testing, and analytical chemistry.
Corrosion Research
Imagine your favorite bike slowly rusting away. That's corrosion in action - a nightmare for anything metal. Potentiostats are like superheroes in the fight against corrosion. They can simulate different environments to see how metals react and help us understand why they corrode. This knowledge is super important for making materials that can stand up to harsh conditions. We're talking about saving industries billions by helping them choose the right materials from the get-go.
Chemical Synthesis
Chemical synthesis is like cooking, but instead of food, we're making new chemicals. Potentiostats help us understand the recipe - the step-by-step process of a chemical reaction. By controlling the conditions and measuring the system's response, scientists can figure out the best way to make new chemicals. This could mean faster, safer, and more environmentally friendly processes for making everything from medicines to materials.
Battery Testing
In our quest for green energy, batteries are the star players. Potentiostats act as their coaches, testing their limits to see how much energy they can store and how many times they can be recharged before they wear out. This is crucial for designing better batteries for everything from your phone to electric cars. With climate change breathing down our necks, understanding batteries better means we can rely less on fossil fuels.
Analytical Chemistry
In analytical chemistry, potentiostats help us measure the tiny currents that flow as a result of chemical reactions. This can tell us a lot about the substances involved. For example, they can be used to develop sensors that monitor health markers in sweat or environmental pollutants in water. It's like having a lab on a chip, giving us real-time data about the world around us and inside us.
To sum it up, potentiostats are not just pieces of equipment. They're gateways to understanding the electrochemical processes that underpin so much of modern science and technology. From protecting our infrastructure and making better batteries to uncovering the secrets of chemical reactions, potentiostats help us navigate the complex dance of electrons that power our world.
Remember that the applications of potentiostats are as diverse as they are impactful, shaping a future where our energy is cleaner, our materials last longer, and our understanding of chemistry is deeper.
Key Differences and Comparisons
Diving deeper into electrochemistry, we encounter several devices that, while similar, serve distinct purposes. Understanding the differences between a potentiostat, galvanostat, potentiometer, and impedance analyzer can be like figuring out which key fits which lock. Let's simplify this.
Potentiostat vs. Galvanostat
Potentiostat:
Focus: Controls the potential (voltage) across an electrochemical cell.
Use Case: Ideal for experiments where maintaining a constant potential is crucial, such as studying the kinetics of a chemical reaction.
Galvanostat:
Focus: Controls the current flowing through an electrochemical cell.
Use Case: Perfect for experiments that require a constant current, like electroplating or battery charge/discharge testing.
Many modern devices combine both functionalities, allowing researchers to switch between controlling potential and current based on their experimental needs.
Potentiostat vs. Potentiometer
Potentiostat:
Input Impedance: High, to avoid drawing current from the cell.
Potential Range: Can be broad, determined by the instrument's design and the electrochemical system's needs.
Current Range: Measures current as a response to the applied potential, offering insights into the electrochemical reaction's dynamics.
Potentiometer:
Input Impedance: Also high, but used for different purposes like measuring the potential without supplying or drawing significant current.
Potential Range: More limited, often used for simpler measurements like pH or basic electrochemical cell potentials.
Potentiostat vs. Impedance Analyzer
Impedance Analyzer:
Sine Wave Profile: Applies a sine wave potential and measures the response, providing detailed information on the impedance and phase shift.
Impedance Measurement: Offers insights into the electrochemical system's resistance and reactance, crucial for understanding battery performance, corrosion, and material properties.
Phase Shift Calculation: Helps in deciphering the capacitive and inductive elements of an electrochemical cell, informing on the kinetics and mechanism of the reactions.
In contrast, the potentiostat is more focused on controlling and measuring the direct effects of potential or current, without directly analyzing the frequency response as the impedance analyzer does.
By understanding these key differences, researchers can select the right tool for their specific needs, whether they're delving into the minutiae of chemical reactions, designing better batteries, or exploring new materials. Each instrument, from potentiostats to impedance analyzers, plays a unique role in unraveling the complexities of electrochemistry, paving the way for innovations that touch every corner of our lives.
Frequently Asked Questions about Potentiostats
Navigating electrochemistry can sometimes feel like trying to read a map without any landmarks. Potentiostats are one of those tools that pop up often, but what exactly are they, and how do they differ from their electrochemical cousins? Let's break it down with some simple answers to common questions.
What is the difference between potentiostat and galvanostat?
Imagine you're at the helm of a ship. As a captain, you have two main ways to navigate: by setting a specific direction (like north or east) or by following a specific path laid out in the water.
Potentiostat: This is like setting your ship to always head north, no matter what. In scientific terms, a potentiostat controls the potential (or voltage) across an electrochemical cell. It decides the "direction" and keeps it steady, letting the current (the ship's speed) adjust as needed based on the "waters" or reactions happening in your experiment.
Galvanostat: Now, imagine instead you decide to follow a path in the water, maintaining a constant speed no matter which direction you're heading. A galvanostat keeps the current constant, letting the potential (or direction) change as needed.
Potentiostats control the direction (voltage), while galvanostats control the speed (current).
How does a potentiostat control potential?
Let's go back to our ship analogy. Controlling your direction requires a steady hand and an eye on the compass, making adjustments as needed to stay on course.
In a lab, a potentiostat does something similar for electrochemical experiments. It uses a system of feedback loops to "look at the compass" -- that is, it constantly checks the voltage between two electrodes. If the "ship" starts to drift off course (the voltage changes), the potentiostat "turns the wheel" (adjusts the current) to get back to the right direction.
This process involves three main players:
Working Electrode: Where the reaction you're interested in happens.
Reference Electrode: Acts like the compass, providing a stable voltage for comparison.
Counter Electrode: Completes the circuit, allowing current to flow.
By maintaining the potential at a set level, researchers can observe how reactions change under specific conditions, much like seeing how our ship behaves when heading north in calm versus stormy seas.
What are the applications of a potentiostat?
Potentiostats are the Swiss Army knives of the electrochemical research world. They're used in a variety of applications, each taking advantage of their ability to precisely control and measure reactions. Here are a few key areas:
Corrosion Research: Understanding how materials degrade over time, helping us build longer-lasting structures.
Chemical Synthesis: Creating new chemical compounds, possibly leading to new drugs or materials.
Battery Testing: Improving battery life and safety by studying how they charge and discharge.
Analytical Chemistry: Detecting and quantifying substances, from pollutants in water to metals in our food.
In short, potentiostats help us understand the invisible reactions that shape our world, from the rust on a bridge to the battery in your smartphone. They're essential tools in our quest to make materials last longer, reactions more efficient, and our environment cleaner.
By answering these questions, we've only scratched the surface of what potentiostats can do. But hopefully, you now have a clearer map to navigate the complex but fascinating world of electrochemistry. Next up, we'll dive deeper into how Micantis is revolutionizing the field with cutting-edge potentiostat technology, streamlining battery testing, and analysis for researchers and industries alike.
Conclusion
Micantis: Streamlining Battery Testing and Analysis
In the intricate world of electrochemistry, potentiostats play a pivotal role. They're the silent heroes behind the development of better batteries, the guardians of our metal structures against corrosion, and the key to unlocking new chemical reactions. But with the challenges of modern battery testing and analysis, the need for advanced, user-friendly, and efficient tools has never been greater. This is where Micantis shines.
Micantis is not just another name in the field; it's a game-changer. Our platform and potentiostat technology are designed with one goal in mind: to make your research as straightforward and impactful as possible. We understand the hurdles researchers face—from the tedious task of data management to the intricate dance of optimizing battery performance. That's why we've put our best minds to work, creating solutions that not only meet these challenges but turn them into opportunities for innovation.
Streamlining Data Management
Imagine conducting an experiment and having all your data automatically organized, analyzed, and ready for interpretation. With Micantis, this isn't a dream—it's your new reality. Our platform seamlessly integrates with our potentiostat technology, ensuring that your data flows smoothly from the lab bench to your screen without the headache of manual entry or the risk of errors. This means you can spend more time on what matters most: pushing the boundaries of what's possible.
Empowering Battery Performance Analysis
The battery market is evolving rapidly, driven by the demand for more sustainable and efficient energy solutions. At Micantis, we're at the forefront of this evolution, providing tools that enable precise control over applied potential and deep insights into battery behavior. Whether you're developing the next generation of electric vehicle batteries or optimizing energy storage for renewable sources, our technology gives you the edge you need to succeed.
A Partner in Innovation
At Micantis, we believe in the power of collaboration. We're not just a provider of high-tech potentiostats; we're your partner in the journey of discovery. Our team is dedicated to supporting your research with not only our technology but also our expertise. Whether you're a seasoned electrochemist or just starting out, we're here to help you achieve your goals and make a lasting impact on the world.
The Future of Electrochemistry
As we look to the future, it's clear that potentiostats will continue to play a vital role in advancing our understanding of electrochemical processes and developing new technologies. With Micantis, you have a partner that's committed to innovation, offering advanced solutions that simplify the complex, streamline the cumbersome, and enhance the performance of your research.
In conclusion, the integration of Micantis potentiostat technology and our comprehensive analysis platform is revolutionizing the field of electrochemistry and battery testing. By providing precise control, efficient data management, and powerful analysis tools, we're paving the way for the next generation of breakthroughs. Explore the future of electrochemistry with Micantis, and take your research to new heights.
Join us at Micantis, and let's write the next chapter of electrochemical research together.